Breast Cancer Imaging
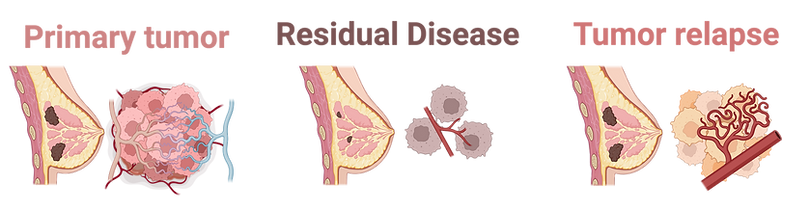
From Detection to Recurrence Prevention in Breast Cancer
With the widespread adoption of mammograms for cancer detection, modern research has principally pivoted towards a focus on how to predict recurrence and ultimately to prevent it. Approximately 30% of breast cancer patients lacking any clinical or pathological signs of metastasis have disseminated tumor cells present at the time of their diagnosis. Thus, residual disease at the primary tumor site after therapy could be viewed as a surrogate for micro-metastasis elsewhere in the body that may have evaded the drug and could potentially return as recurrent disease.
Residual disease at the primary tumor site after systemic therapy could be viewed as a surrogate for micro-metastasis elsewhere and an important predictor for recurrence.
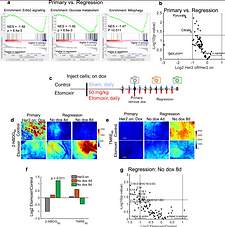
Given that recurrence is an important determinant of clinical outcome, it is important to predict and mitigate the presence of residual disease. We have developed a metabolic biomarker imaging technology, the CapCell to report on how tumors reshape their metabolic needs to evade therapeutic stress. Through spatial and longitudinal imaging, the CapCell provides provides a window into metabolic reprogramming of regression, residual disease and recurrence in patient xenograft and genetically engineered mouse models.
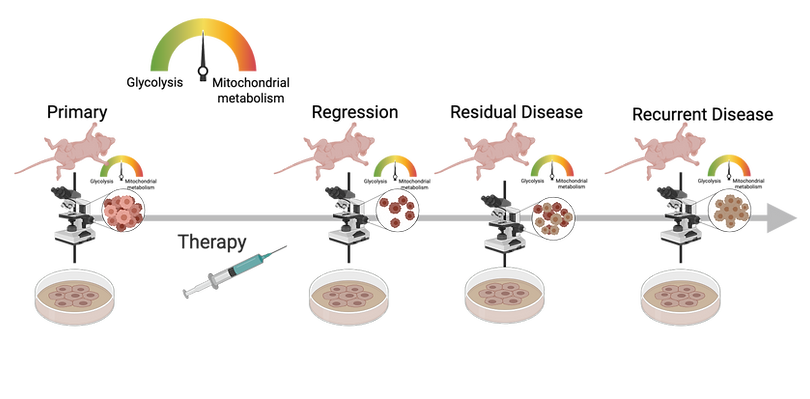
We are designing tools to quantify the metabolic and vascular changes that occur at these points to provide an unprecedented window into metabolic reprogramming of residual disease and recurrence. Using both in vitro organoid imaging and in vivo imaging of genetically engineered and patient-derived tumors, we can image and understand how these tumors evade therapeutic stress
Breast tumors use glucose and fatty acids as nutrient sources. Energy can be generated from either glycolysis or mitochondrial metabolism (oxidative phosphorylation or OX PHOS). CapCell imaging shows that resilient tumor cells survive by switching from a predominantly glycolytic phenotype to mitochondrial metabolism. This metabolic flexibility and heterogeneity are minimal or absent in treatment responsive disease. This points to the importance of spatial and longitudinal metabolic imaging as a way to inform treatment strategies that can mitigate tumor relapse.
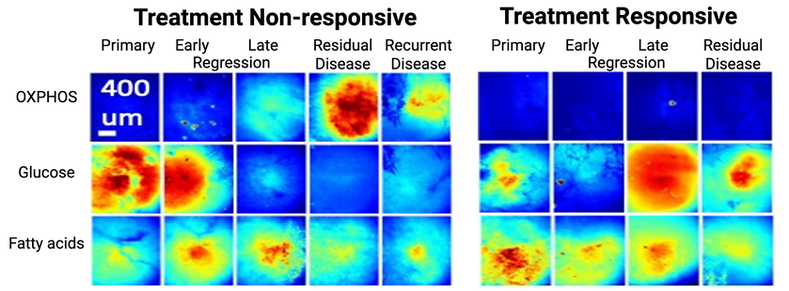
The CapCell can detail the differences in metabolic programming associated with treatment responsive and treatment non-responsive disease (OX PHOS - oxidative phosphorylation).
GWHT Breast Cancer Imaging Research Team
Collaborators
Published Research
.png)








_1.jpg)